HealthEcon, based in Basel, Switzerland, is a consulting firm for Health Technology Assessment, European market access and value strategy for the pharmaceutical industry.
- We are your one-stop-partner for European market access.
- We advise you on how to bridge the gap between regulatory and reimbursement requirements.
- Our experience helps you to structure your internal processes and teams.
- We provide guidance on the targeted use of real world data.
Focus
HealthEcon is one of the most experienced healthcare consulting companies in Europe. We are based in Basel, Switzerland, with more than 1100 projects completed since our establishment in 1981. We mainly provide consultation services on pharmaceuticals for the leading European markets, including
- reimbursement and pricing analyses,
- early advice on market access and trial design on national and european level,
- appraisal of clinical evidence and healtheconomic assessment,
- AMNOG dossiers
Services are provided from our Basel office.
If required, HealthEcon staff also temporarily works on-site with clients’ project teams at their regional offices.
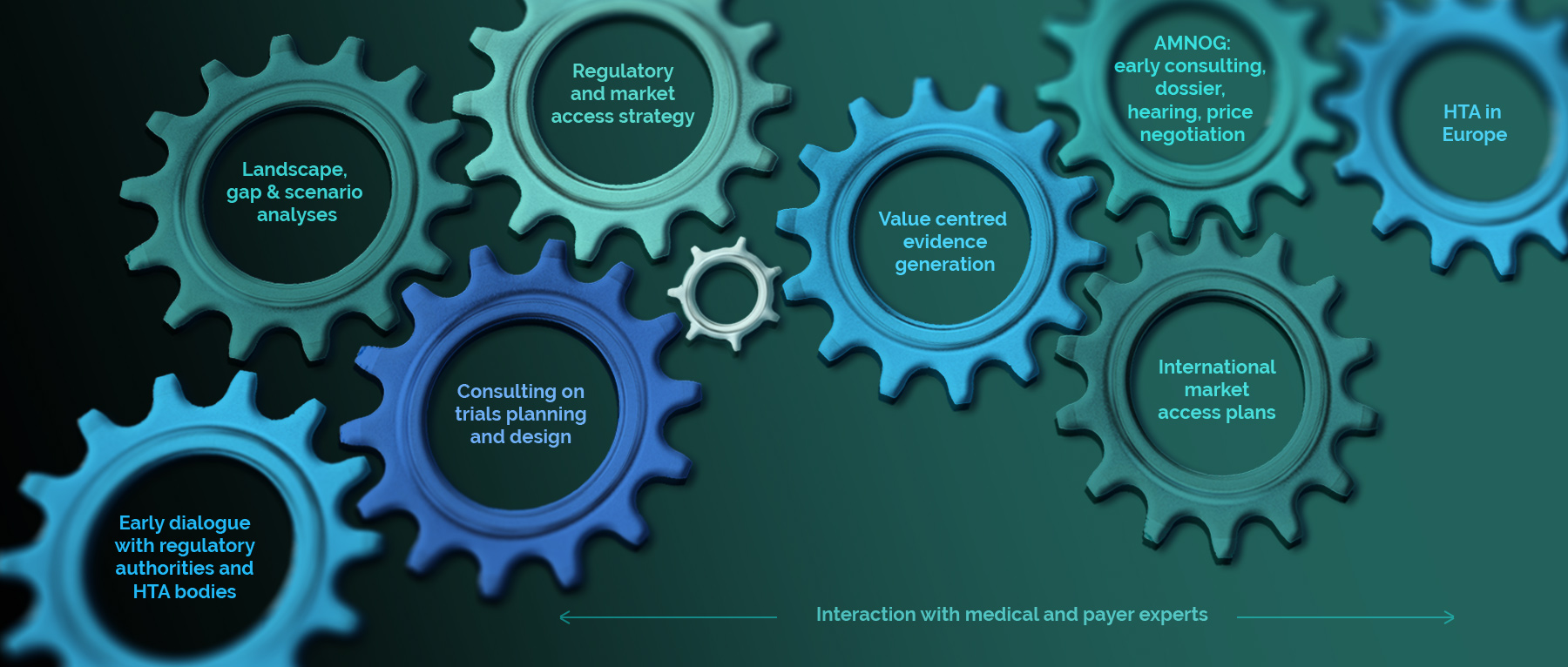
Early dialogue with regulatory authorities and HTA bodies
- Early advice meetings, parallel consultation and joint advice
- Compilation of consultation requests
- Supporting client before and during actual consultation meetings
Landscape, gap & scenario analyses
- Identifying the product’s profile compared to…
- regulatory requirements
- market access requirements
- competitors
- Identifying gaps & proposing potential remedies
- Assessing & prioritizing remedies
Regulatory & market access strategy
- Comparison of regulatory strategy with market access requirements
- Aligning regulatory & payer strategy
- Compilation of reimbursement dossiers
Consulting on trial planning and design
- Shaping clinical trial designs:
- Target patient population
- Relevant endpoints
- Subgroups & specific populations
- Types of analyses
- Quality assessments of clinical trials (e.g. CONSORT)
Value centred Evidence generation
- Statistical Analysis Plans
- Literature search and reviews
- Meta-Analyses
- Determination of unmet medical need
- Healtheconomic Evaluation (cost-efffectiveness, budget impact, cost-utility, cost-benefit)
AMNOG (Germany): early consulting, dossier, hearing, price negotiation
- Preparation of waivers
- Early advice meetings
- Prepare product specific strategy (e.g. orphan status, portfolio analyses)
- Compile AMNOG Dossiers
- Oral hearings
- Development of pricing strategy and outline of arbitration board strategies
International Market Access Plans
- Developing international access plans.
- Modular preparation of data:
- Core clinical data
- Country specific supplements:
- Medical unmet need & epidemiology
- Patient relevant outcomes
- Subgroups
- Healtheconomic
- Preparation of global value dossiers (GVD)
- Adaption / regional staffing of international models
HTA in Europe
- Relevance of activities on EU level
- EUNetHTA core models
- Early Advices
- Parallel consultation
Interaction with MEDICAL AND PAYER experts
- Setting up key messages for medical and payer stakeholders
- Setting up medical & payer Adboards
- Conducting payer research incl. payer face to face interviews
- Developing congress presentations and full length publications
Projects
Since our establishment in 1981, we have completed more than 1100 projects for over 200 clients. We have supported European governments, associations, sickness funds and pharmaceutical companies. Our clients include most of the global top 20 pharmaceutical companies, as well as small and medium-sized biotech companies.
Reference are available upon request.
Kontakt
HealthEcon® AG
Steinentorstrasse 19
4051 Basel
Switzerland
+41 (0)61-284 95 60
info@healthecon.com
www.healthecon.com